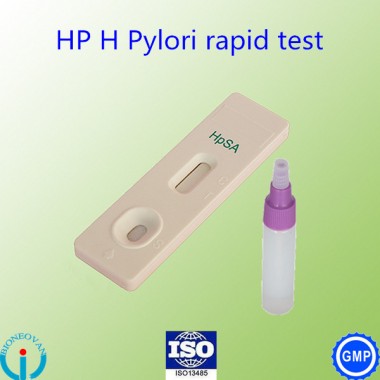
High Quality H. Pylori Antigen rapid Test kits / HP test (Colloidal Gold)
Product Description
Hp test kit is an in vitro qualitative immunochromatographic assay for the rapid detection of Helicobacter pylori antigens in human stool specimen. The test results are intended to aid in the diagnosis of H. pylori infection, to monitor the effectiveness of therapeutic treatment and to confirm the eradication of H. pylori in peptic ulcer patients.
Hp test kit is a sandwich solid phase immunochromatographic assay. To perform the test, an aliquot of diluted stool sample is added to the sample well of the test cassette. The sample flows through a label pad containing H. pylori antibody coupled to red-colored colloidal gold. If the sample contains H. pylori antigens, the antigen will bind to the antibody coated on the colloidal gold particles to form antigen-antibody-gold complexes. These complexes move on the nitrocellulose membrane by capillary action toward the test line region on which H. pylori specific antibodies are immobilized. As the complexes reach the test line, they will bind to the antibody on the membrane in the form of a line. A second red control line will always appear in the result window to indicate that the test has been correctly performed and the test device functions properly. If H. pylori antigen is not present or lower than the detection limit of the test, only the control line will be visible. If the control line dose not developed, the test is invalid.
Stool specimens should be collected in containers that do not contain media, preservatives, animal serum or detergents as any of these additives may interfere with the HP Stool Ag Test. Specimens may be stored at 2-8°C for 3 days without interfering with the assay performance. For long-term storage of specimens, -20°C or colder is recommended. Repeated freezing and thawing of specimens is not recommended and may cause erroneous results. Do not store specimens in self-defrosting freezers.
Preparations:
Allow all reagents and samples to equilibrate to room temperature before proceeding with the test.
Write the specimen number on the cassette and sample preparation device.
Procedure:
1. Remove sample probe from preparation device and coat liberally with sample. Replace probe in vial and shake to disperse solid material.
2. For liquid or semi-solid stools, 100µl of stool may be added using an appropriate pipette.
3. Allow the mixture to stand for 1 -2 minutes.
4. Snap top from preparation device and invert. Add 2 – 3 drops (100µl) to the sample well of the test cassette.
5. Leave the test at room temperature and read the results between 5 and 15 minutes after addition of sample. Note: Weak and negative samples may require the full 15 minutes to develop.




Packaging & Shipping
Hp Rapid test kit
S/N | Feature | Details |
1 | Shelf life: | 24 months |
2 | Storage: | room temperature |
3 | Specimen: | feces/serum |
4 | Package: | 40T(4mm)/box |
5 | MOQ: | 10kit |
6 | Manufature | OEM service |
7 | Packaging Details: | Express for export |
8 | Lead Time: | 2-3 weeks after payment |
9 | Payment Terms: | T/T, Western Union, PayPal or Money Gram |
10 | Supply Ability: | 100 000 kits/Month |
11 | Place of Origin: | Beijing, China (Mainland) |
12 | Brand Name: | BIONEOVAN |
13 | Certification: | ISO13485 & CFDA & GMP |
Our Services
1. Experience: 10 years of experience in R&D and distributing advanced in-vitro medical diagnostic products.
2. Quality: Our products are with ISO9001, ISO13485,GMP
3. Service: Including pro sale and after sale service and timely reply via email, phone.etc.
4. Price: You can get first -hand prices from our factory.
5. Delivery: On time delivery. 10~20 days after payment.
Company Information
Bioneovan was built in 1988 in Beijing, China and was the earliest domestic high-tech enterprise which was specialized in the field of ELISA TESTS & RAPID TESTS as both supplier and manufacturer with GMP, ISO9001:2008, and ISO13485 Certificates.
Our products mainly go through the following tests:
1.Elisa : Hbsag elisa kit, Hbsab, Hbeag, Hbeab, Hbcab, Anti-HCV, HAV igm, HEV Igm, HEV Igg, Anti-HIV1&2, Anti-hiv Ab/Ag elisa test kit and so on.
2. Rapid test : Hbsag cassette hbsag strip, hcv rapid test cassette, hcv rapid test strip, hev igm cassette, hev igg cassette, hav igm cassette, h-pylori ag cassette and other 40 kinds of rapid test cassette or strip.
Basic Information Indicates information has been verified onsite by a certification specialist
| Product/Service (We Sell): | Medical Test Kit (elisa Test Kit, Rapid Test Kit for the Infectious Disease) |
| Brands: | Bioneovan |
| Number of Employees: | 101 - 300 People |
Trade & Market
| Main Markets: | Southern Europe
Central America
Mid East
Eastern Asia
Northern Europe
Western Europe
South Asia
North America
Southeast Asia
South America
Eastern Europe
Africa
Domestic Market |
| Total Annual Sales Volume: | US$2 Million - US$5 Million |
| Export Percentage: | 5% - 15% |
Factory Information
| Factory Size (Sq.meters): | 5,000-10,000 square meters |
| Factory Location: | No.18 Keyuan Road Daxing Industry Zone,Beijing, China No.18 Keyuan Road Daxing Industry Zone,Beijing, China |
| Number of Production Lines: | 2 |
| Number of R&D Staff: | 31 - 40 People |
| Number of QC Staff: | 11 - 20 People |
| Management Certification: | ISO13485 ISO9001, GMP, FSC |
| Contract Manufacturing: | OEM Service Offered |