hiv rapid test kit/Aids rapid test kit
- FOB Price:Get Latest Price >
- Min.Order:1000 Piece(s)
- Production Capacity: 20000 pcs/week
- Payment Terms:T/T , PayPal , Money Gram
- Favorite
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


Bioneovan Co.,Ltd
Bioneovan is specialized in ELISA & RAPID TESTS as both supplier and manufacture since 1988.We have our own factory, so we could provide clients with OEM service and give them Best price.
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank

High Eensitivity Anti-Hiv 1&2 Rapid Cassette/Strip Medical Diagnostic Test Kit
Product DescriptionSpecifications
Pathology laboratory equipments HIV Rapid Test Kit
1.Manufacturer
2.Free Samples
3. Timely delivery
4.High accuracy
SAMPLE REQUEST
The HIV-Card/Strip can be performed using either serum or plasma.
Separate the serum or plasma from blood as soon as possible to avoid hemolysis. Testing should be performed immediately after the specimens have been collected. Do not leave the specimens at
room temperature for prolonged periods. Specimens may be stored at 2-8°C for up to 3 days. For long term storage, specimens should be kept below -20°C.
Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Specimens should not be frozen and thawed repeatedly.If specimens are to be shipped, they should be packed in compliance with federal regulations for transportation of etiologic agents.
TEST PROCEDURE:
1. When you are ready to begin testing, open the sealed pouch by tearing along the notch. Remove the test from the pouch.
2. Use the disposal pipette inside the pouch, add about 150ul sample into the Sample Well of the card/srip.
3. Wait 5-30 minutes and read results. Do not read results after 30minutes.
4. Attention: The operation procedure change a little, this can make the sensitivity is better.
STORAGE AND STABILITY
Store at 4~30°C, dry places for 24 months. Use test card within 1 hour once open to atmosphere when the humidity is below 60%, or use it immediately if the humidity is higher.

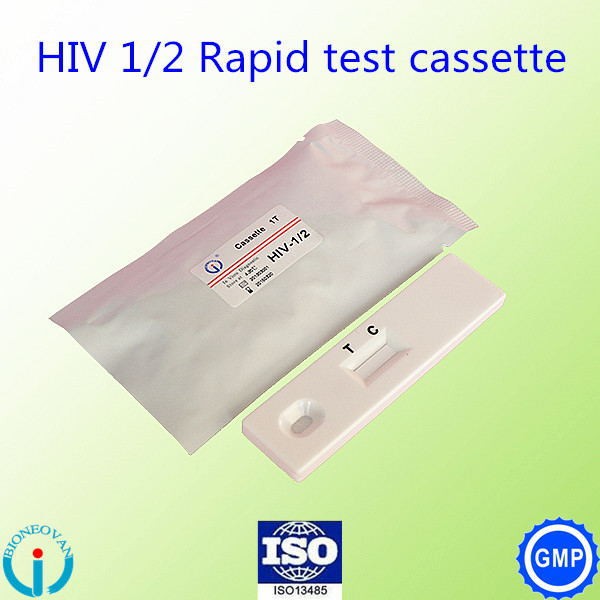
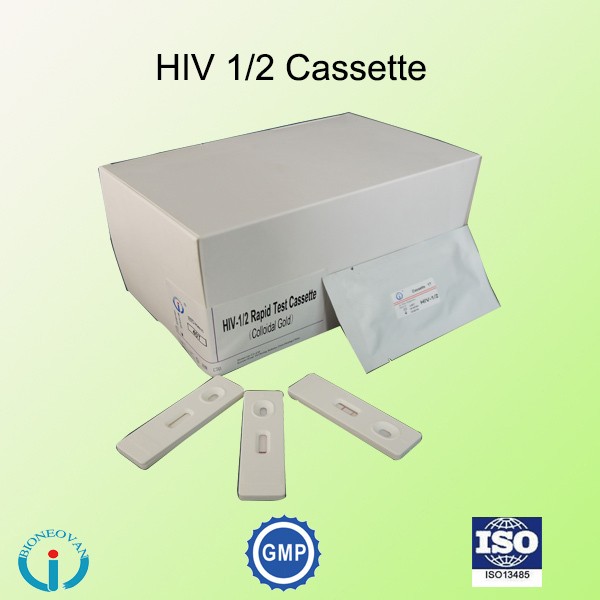



Packaging & Shipping
HIV Laboratory Reagents
S/N | Feature | Details |
1 | Shelf life: | 24 months |
2 | Storage: | 4~30°C room temperature |
3 | Specimen: | Serum |
4 | Package: | 40 tests/kit |
5 | MOQ: | 10 kit |
6 | Manufature | OEM service |
7 | Packaging Details: | Express for export |
8 | Lead Time: | 2-3 weeks after the payment |
9 | Payment Terms: | T/T, Western Union, PayPal or Money Gram |
10 | Supply Ability: | 100 000 kits/Month |
11 | Place of Origin: | Beijing, China (Mainland) |
12 | Brand Name: | BIONEOVAN |
13 | Certification: | ISO13485 & CFDA & GMP |
Our Services
1.Experience: 10 years of experience in R&D and distributing advanced in -vitro medical diagnostic products.
2. Quality: Our products are with ISO9001, ISO13485,GMP
3.Service: Including pro sale and after sale service and timely reply via email, phone, Skype etc.
4. Price: You can get first -hand prices from our factory.
5. Delivery: Fast delivery. 10~20 days after payment.
Company Information
Bioneovan was built in 1988 in Beijing, China and was the earliest domestic high-tech enterprise which was specialized in the field of ELISA TESTS & RAPID TESTS as both supplier and manufacturer with GMP, ISO9001:2008, and ISO13485 Certificates.
Our products mainly go through the following tests:
1.Elisa : Hbsag elisa kit, Hbsab, Hbeag, Hbeab, Hbcab, Anti-HCV, HAV igm, HEV Igm, HEV Igg, Anti-HIV1&2, Anti-hiv Ab/Ag elisa test kit and so on.
2. Rapid test : Hbsag cassette hbsag strip, hcv rapid test cassette, hcv rapid test strip, hev igm cassette, hev igg cassette, hav igm cassette, h-pylori ag cassette and other 40 kinds of rapid test cassette or strip.
Basic Information Indicates information has been verified onsite by a certification specialist
Product/Service (We Sell): | Medical Test Kit (elisa Test Kit, Rapid Test Kit for the Infectious Disease) | |
Brands: | Bioneovan | |
Number of Employees: | 101 - 300 People |
Main Markets: | Southern Europe | |
Total Annual Sales Volume: | US$2 Million - US$5 Million | |
Export Percentage: | 5% - 15% | |