Bioneovan HEV-IgM ELISA is an in vitro enzyme linked immunoassay supplied for the detection of HEV-IgM in human serum or plasma. It is intended to be used as an aid in supplementary diagnosis to acute hepatitis E infection and prevalence studies among the population.
This kit employs solid phase, capture ELISA method for detection of IgM antibodies to HEV (anti-HEV) in serum or plasma with two-step incubation procedure. Polystyrene microwell is pre-coated with purified activated mouse anti human IgM (μ chain) monoclonal antibody. The HRP conjugated recombinant HEV antigen serves as tracer. TMB is substrate for HRP. The enzyme reaction with substrate TMB produces a color change, and the intensity of the absorbance at 450 nm indicates the presence or absence of Anti-HEV antibodies IgM in the sample. The test is specific, sensitive, reproducible and easy to operate. It is for diagnosis of early infection and epidemic survey.
SAMPLE COLLECTION AND PRESERVATION |
Blood serum samples are routinely prepared form vein. Blood plasma samples are routinely prepared with routine amount of anticoagulant such as heparin or sodium citrate. Sample can be stored at 4°C if tested within two days. Specimens not required within 3 days should be stored at -20°C. Avoid hemolysis and repetitive freeze and thaw of samples. Samples with cloud or precipitation should be centrifugated or filtered before test. Prevent serum from bacterium contamination during collectiing and storing.
1. Bring ELISA Kit for Antibody IgG to Hepatitis E Virus(all reagents), and Specimens to room temperature before use (approximately 30 minutes).
2. Dilute concentrated wash buffer 1:19 with ddH2O.
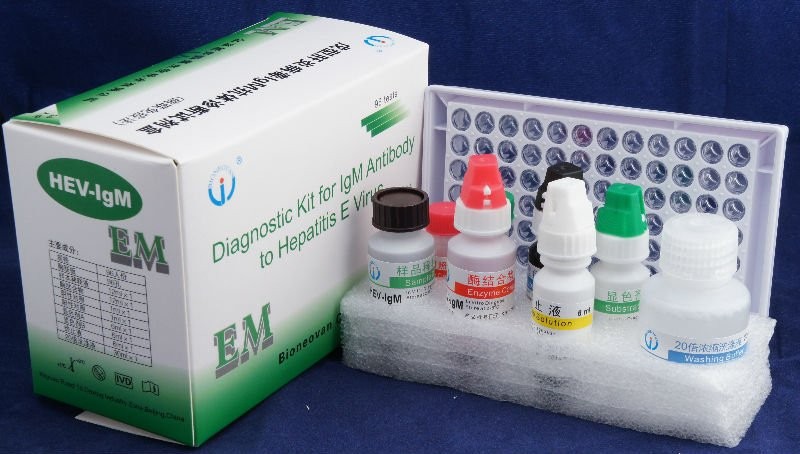
3. For each test, set one blank, two positive and three negative controls. Add 100μl positive and negative control serum into positive and negative control wells respectively.
4. Add 100μl sample diluents in each other test well, then at 10 μl test specimen into the test wells. Pipet up and down to mix the samples well.
5. Cover wells with seal paper, and then incubate 30 minutes at 37°C.
6. Discard the liquid in all wells and fill the wells with wash solution. Lay aside for 15 seconds, discard the liquid in all wells and fill the wells with wash solution. Repeat 5 times and dry wells after last wash.
7. Add 100 μl Enzyme Conjugant in each well except the blank.
8. Cover wells with seal paper, and then incubate 30 minutes at 37°C.
9. Repeat step 6.
10. Add 50μl substrate A and B respectively to each well including the blank well. Mix gently, protected from light and incubates 15 minutes at 37°C.
11. Add 50μl of stop solution into each well to stop the reaction, including blank well.
12. Measure the absorbance at 450 nm against the blank, or measure the absorbance at 450 nm/630-690 nm.
The testing is for qualitative and assistant diagnosis. Confirmation of infection should refer to the clinical and other diagnosis.
If the OD of positive controls is not below 1.0, OD of negative is not higher than 0.1, the assay result is validated. Otherwise, repeat the test.
- 1. The samples should be fresh, avoid hemolysis, bacteria growing and repetitive freeze and thaw.
- 2. Do not exchange reagents from different lots or use reagents from other commercially available kits. The seal paper can’t be used repeatedly.
- 3. Make sure that all reagents are within the validity indicated on the kit box and of the same lot. Never use reagents passed its expiry date.
- 4. Shake reagent gently to mix the reagents well before use. If crystal form in certain reagents, such as wash buffer, it can be used after warming up and mixing well.
- 5. Follow instruction exactly during assay, especially in temperature and time for reactions. All pipetting devices should be used with care and calibrated regularly following the manufacturer’s instructions.
- 6. Put the remained reagents to the sealed pouch, and return to 2~8°C in time.
- 7. To prevent cross-contamination, wear gloves and working suits throughout the procedure, and execute the disinfection and isolation regulations strictly. Dispose of all samples and materials used to perform the test. The 5.0g/L liquid sodium hypochlorite solution or 121°C high pressure steam may be used to disinfect samples and materials before disposal (The positive control serum in the kit has been inactivated already)
96 tests/Kit
Store the kit at 2~8°C.
The shelf life is 12 months. Do not use the kit beyond its expiration date.


Our Services
1. Experience: 10 years of experience in R&D and distributing advanced in -vitro medical diagnostic products.
2. Quality: Our products are with ISO9001, ISO13485,GMP
3. Service: Including pro sale and after sale service and timely reply via email, phone, Skype etc.
4. Price: You can get first -hand prices from our factory.
5. Delivery: Fast delivery. 10~20 days after payment.
Company Information
Bioneovan Co., Ltd. is a leading exporter and manufacturer of diagnostic test kits. We can supply various formats of diagnostic kits on basis of OEM or your brand name. Our products mainly include one step HIV, HCV, HBV, HbsAG TB, Drugs of Abuse, etc. And we can supply Elisa test kits and Blood Grouping too. Our products passed GMP and ISO. Besides, some items also have CE.