Elisa Test Kit Hepatitis Anti-HCV Blood Test Device
Product Description
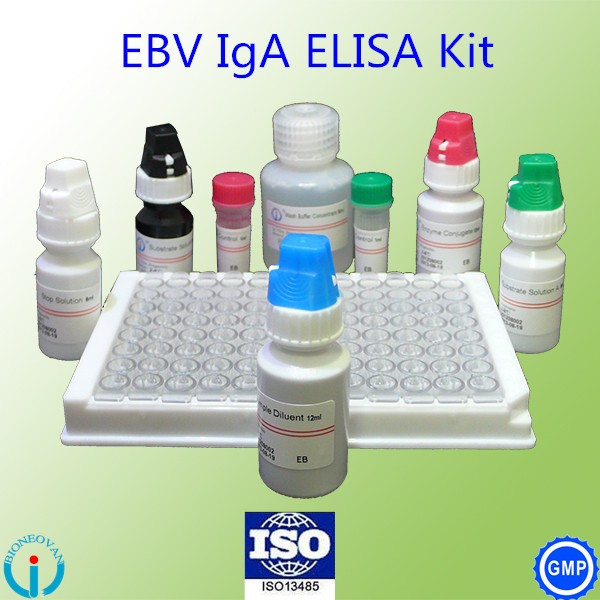
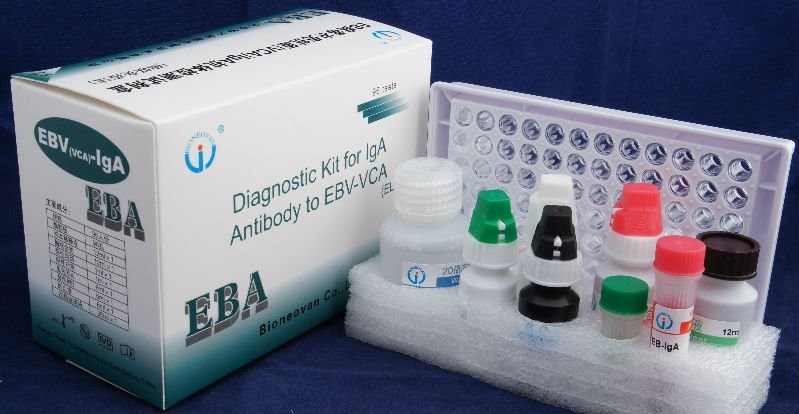
The EBV-VCA IgA Ab ELISA Kit utilizes a detection system where micro plate wells are coated with recombinant antigen corresponding to a highly antigenic segment EBV-VCA. Serum or plasma specimens, controls are added to the wells. During incubation, IgA antibodies specific for EBV-VCA present in the specimen will bind to the recombinant antigen fixed onto the micro plate wells. The wells are washed to remove unbound materials, and anti-human IgA peroxides conjugate will bind to the antigen-antibody complex and excess unbound enzyme conjugates are again removed by washing. The enzyme substrate, tetramethylbenzidine (TMB), is added upon incubation the substrate will be hydrolyzed by the bound enzyme and a blue or blue-green color develops in wells containing EBV-VCA IgA antibodies. The enzyme reaction is stopped by the addition of sulphuricacid. The intensity of color developed is read spectrophotometrically at 450nm(450 nm/630 nm) and is proportional to the amount of antibodies present in the specimen.
| SPECIMEN COLLECTION AND PREPARATION |
Serum should be prepared from a whole blood specimen obtained by acceptable medical techniques. Either serum or plasma can be used in this test. Remove serum or plasma from the clot or blood cells as soon as possible to avoid hemolysis. Specimen with extensive particulate should be clarified by centrifugation prior to use. Specimen frozen at -20°C or colder may be used. Avoid repeated freeze thaw.
Unopened test kits should be stored at 2-8°C upon receipt and the micro titer plate should be kept in a sealed bag to minimize exposure to damp air. Use up the reagents as soon as possible after the kit is unpacked.
Packaging & Shipping
EBV blood test device
|
S/N | Feature | Details |
1 | Shelf life: | 12 months |
2 | Storage: | 2-8°C No freezing |
3 | Specimen: | Serum |
4 | Package: | 96 test/kit |
5 | MOQ: | 10 kits |
6 | Shipping : | By air |
7 | Packaging Details: | Express for export |
8 | Lead Time: | 2-3 weeks after the payment |
9 | Payment Terms: | T/T, Western Union, PayPal or Money Gram |
10 | Supply Ability: | 100 000 kits/Month |
11 | Place of Origin: | Beijing, China (Mainland) |
12 | Brand Name: | BIONEOVAN |
13 | Certification: | ISO13485 & CFDA & GMP |


Our Services1: Competitive price with high quality.
2: Original manufacturer provide OEM service.
3:on time delivery near beijing and tianjin Port.
4: Free samples available for your evalutaiton.
5: ISO 9001,ISO13485,GMP,Medical devices-quality management system certificate
Company Information
Bioneovan was built in 1988 in Beijing, China and was the earliest domestic high-tech enterprise which was specialized in the field of ELISA TESTS & RAPID TESTS as both supplier and manufacturer with GMP, ISO9001:2008, and ISO13485 Certificates.
Our products mainly go through the following tests:
1.Elisa : Hbsag elisa kit, Hbsab, Hbeag, Hbeab, Hbcab, Anti-HCV, HAV igm, HEV Igm, HEV Igg, Anti-HIV1&2, Anti-hiv Ab/Ag elisa test kit and so on.
2. Rapid test : Hbsag cassette hbsag strip, hcv rapid test cassette, hcv rapid test strip, hev igm cassette, hev igg cassette, hav igm cassette, h-pylori ag cassette and other 40 kinds of rapid test cassette or strip.
Basic Information Indicates information has been verified onsite by a certification specialist
| Product/Service (We Sell): | Medical Test Kit (elisa Test Kit, Rapid Test Kit for the Infectious Disease) |
| Brands: | Bioneovan |
| Number of Employees: | 101 - 300 People |
Trade & Market
| Main Markets: | Southern Europe
Central America
Mid East
Eastern Asia
Northern Europe
Western Europe
South Asia
North America
Southeast Asia
South America
Eastern Europe
Africa
Domestic Market |
| Total Annual Sales Volume: | US$2 Million - US$5 Million |
| Export Percentage: | 5% - 15% |
Factory Information
| Factory Size (Sq.meters): | 5,000-10,000 square meters |
| Factory Location: | No.18 Keyuan Road Daxing Industry Zone,Beijing, China No.18 Keyuan Road Daxing Industry Zone,Beijing, China |
| Number of Production Lines: | 2 |
| Number of R&D Staff: | 31 - 40 People |
| Number of QC Staff: | 11 - 20 People |
| Management Certification: | ISO13485 ISO9001, GMP, FSC |
| Contract Manufacturing: | OEM Service Offered |

FAQ
Q:Can I get a sample before bulk order? How can I get it?
A:Sample is free, but freight collect.
You could prepay the freight cost by PayPal or Western union, we will send out sample ASAP once getting your payment.
Q: Do you have minimum order requirements?
A: No, according to client’s need.
Q: Can we visit your factory?
A: Certainly. Welcome your visit.
Q: How to ensure the cargo quality?
A: We will supply bulk sample before shipping. They can represent the cargo quality.
Q: Why choose Bioneovan?
A: We are the professional factory not trading company, so the price is quite competitive in the market.
We have ISO13485 & CFDA & GMP certificate. Products quality can be guaranteed.
Q: What’s the payment method?
A: T/T is highly appreciated . Accept USD.
Western Union/Money Gram/PayPal: Quickly to the account, Priority in delivery
Behalf Paying: Your Chinese friends or your Chinese agent can pay for in RMB.